D. Aromatic Hydrocarbons Pdf
Posted : admin On 20.09.2019Urinary polycyclic aromatic hydrocarbon (OH-PAH) metabolite concentrations and the effect of GST polymorphisms among US Air Force personnel exposed to jet fuel Rodrigues EG, Smith K, Maule AL, Sjodin A, Li Z, Romanoff L, Kelsey K, Proctor S, McClean MD.
Naming aromatic compounds: This nomenclature tutorial video takes you through the IUPAC rules for benzene type molecules and includes the common names for substituted benzene. Aromatic Compounds with a Single SubstituentWhen there is a single substituent on a benzene ring and the substituent contains six or fewer carbons, the substituent is included as a prefix to benzene. Alkyl groups are named according to the alkane series convention ending with -yl: methyl (for a single carbon), ethyl (for two carbons), propyl (for three carbons), etc.If the substituent contains more than six carbons, the alkane portion is named first, and the aromatic ring portion is added as a suffix. For instance, an aromatic ring bonded to an 8-carbon chain would be 1-phenyloctane, and not octylbenzene.
Free house cheat sims 4. Enjoy!How To Use Sims 4 CheatsIn order to use Sims 4 cheats, you’ll need to navigate to the cheats console.
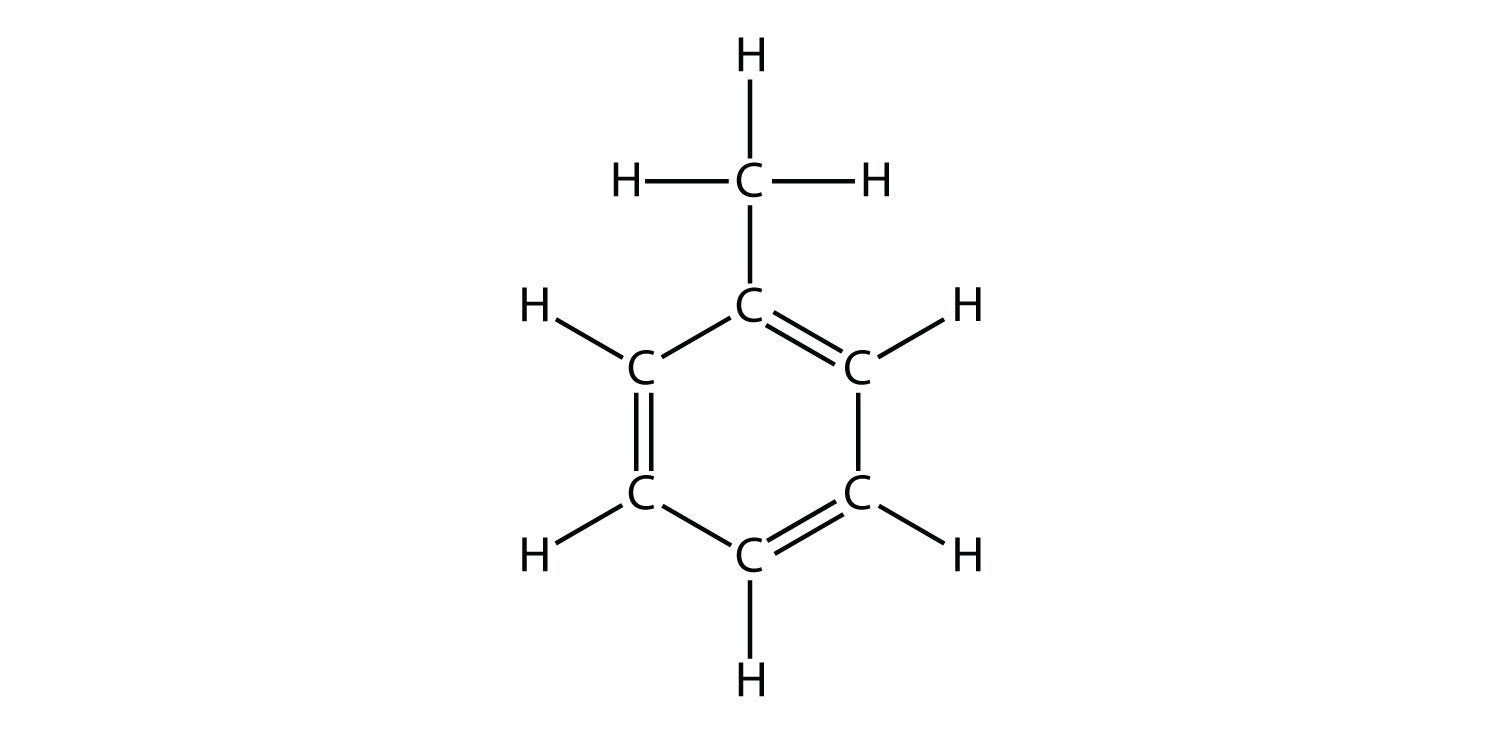
Aromatic Compounds with Multiple SubstituentsWhen there are multiple substituents, ring atoms are numbered to minimize the numbers assigned to the substituted positions.Disubstituted benzene rings can be named based on the relative positions of the substituents: the prefix ortho– is used if the substituents occupy adjacent positions on the ring (1,2), meta– is used if the substituents are separated by one ring position (1,3), and para– if they are found on opposite sides of the ring (1,4). Benzene: Benzene can only be fully depicted with all of its resonance structures, which show how its pi-electrons are delocalized throughout its six-carbon ring. This delocalization leads to a lower overall energy for the molecule, giving it greater stability. Structure of Aromatic CompoundsAromatic compounds are cyclic structures in which each ring atom is a participant in alatexpi/latex bond, resulting in delocalized latexpi/latex electron density on both sides of the ring. Due to this connected network of latexpi/latex bonds, the rings are planar, unlike the boat or table structures typical of cycloalkanes.
Physical Properties of Aromatic CompoundsAromatic compounds are generally nonpolar and immiscible with water. As they are often unreactive, they are useful as solvents for other nonpolar compounds.
Due to their high ratio of carbon to hydrogen, aromatic compounds are characterized by a sooty yellow flame. Reactivity of Aromatic Compounds. Electrophilic Aromatic Substitution: The electron-rich benzene makes a bond with an electron-deficient chemical species (E +, the electrophile) which takes the place of an H-atom in the original structure.
The reaction preserves the pi system of electrons and therefore the aromatic character of the benzene ring.The double bonds in aromatic compounds are less likely to participate in addition reactions than those found in typical alkenes. Instead, cyclic aromatic compounds undergo electrophilic substitution reactions (reactions in which the ring acts as an nucleophile to a suitable electrophile). When benzene participates in such substitution reactions, the product retains the stability associated with the aromatic latexpi/latex electron system. This stability is lost in electrophilic addition because the product is not aromatic.
Sources of Aromatic CompoundsAromatic compounds are produced from a variety of sources, including petroleum and coal tar. Poly-aromatic hydrocarbons are components of atmospheric pollution and are known carcinogens. Aromatic compounds are also interesting because of their presumed role in the origin of life as precursors to nucleotides and amino acids. Aromatic substitution: Example of an aromatic substitution reaction. The double bond attacks the NO2 cation, then a proton (hydrogen cation) is lost to rearomatize the system. Nucleophilic Aromatic SubstitutionsIn a nucleophilic aromatic substitution reaction, a nucleophile displaces a substituent on an aromatic ring.
The replaced species is typically a good leaving group, like nitrogen gas or a halide ion. The presence of an electron-withdrawing group on the ring can speed up the progress of this class of reactions. Chemically, this is similar to an addition reaction to a Michael acceptor or other electron-deficient, unsaturated system, followed by an elimination reaction. Electrophilic Aromatic Substitutions. Electrophilic aromatic substitution (EAS): This reaction mechanism takes place from bottom to top.
EAS occurs ortho or para to electron donating groups, such as amines, due to the stabilization of the intermediate positive charge. The four structures drawn in the middle of the diagram are all resonance structures.
Due to the electrons provided by the NH2 group, this intermediate is stabilized, and the para-substitution is favored. As an exercise, draw out the stabilization of the positive charge when ortho substitution occurs.In an electrophilic aromatic substitution reaction, a substituent on an aromatic ring is displaced by an electrophile.
These reactions include aromatic nitration, aromatic halogenation, aromatic sulfonation, and Friedel-Crafts acylations and alkylations. These reactions can involve a resonance-stabilized carbocation intermediate known as a sigma complex. The reactivity can be thought of in terms of an alkene attacking a cationic species, such as in the first step of an acid-catalyzed hydration of an alkene.A number of patterns have been observed regarding the reaction of substituted benzene rings. These observations have been generalized to provide a predictive rule for electrophilic aromatic substitutions. It states that an electron-donating substituent generally accelerates substitution and directs reactivity toward the positions that are ortho and para to it on the ring, while an electron-withdrawing substituent will slow reaction progress and favor the meta position on the ring. Coupling ReactionsCoupling reactions are reactions involving a metal catalyst that can result in the formation of a carbon-carbon bond between two radicals.
D. Aromatic Hydrocarbons Pdf Free
HydrogenationHydrogenation can be used to create a fully saturated ring system. This is similar to the hydrogenation of an alkene to form an alkane, albeit more difficult due to the stability of the aromatic system. CC licensed content, Specific attribution. Aromatic. Provided by: Wikipedia. License:.
ortho. Provided by: Wiktionary. License:. Aromatic hydrocarbon. Provided by: Wikipedia. License:.
Naming aromatic compounds. License Terms: Standard YouTube license. Aromaticity. Provided by: Wikipedia. License:.
aromatic hydrocarbon. Provided by: Wiktionary.
License:. aromaticity. Provided by: Wiktionary. License:. Aromatic hydrocarbon. Provided by: Wikipedia.
License:. Naming aromatic compounds. License Terms: Standard YouTube license. Ortho, meta, and para nomenclature of aromatic compounds. License Terms: Standard YouTube license. Electrophilic-aromatic-substitution-general. Provided by: Wikimedia Commons.
License:. Aromatic. Provided by: Wikipedia.
License:. Aromatic hydrocarbons. Provided by: Wikipedia.
License:. electrophile.
Provided by: Wiktionary. License:. hydrogenation. Provided by: Wiktionary. License:.
nucleophile. Provided by: Wiktionary. License:. Aromatic hydrocarbon. Provided by: Wikipedia. License:. Naming aromatic compounds.
License Terms: Standard YouTube license. Ortho, meta, and para nomenclature of aromatic compounds. License Terms: Standard YouTube license. Electrophilic-aromatic-substitution-general.
Provided by: Wikimedia Commons. License:. Aromatic. Provided by: Wikipedia. License:. Aromatic hydrocarbon.
Provided by: Wikipedia. License:. File:EAS substitution Para director.svg - Wikipedia, the free encyclopedia. Provided by: Wikipedia.
1.8 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.2. ICS CodeICS Number Code 71.040.40 (Chemical analysis); 71.080.20 (Halogenated hydrocarbons)UNSPSC CodeUNSPSC Code 12352005(Aromatic or heterocyclic compounds)Referencing This Standard Link HereLink to Active (This link will always route to the current Active version of the standard.)DOI: 10.1520/D5808-18Citation FormatASTM D5808-18, Standard Test Method for Determining Chloride in Aromatic Hydrocarbons and Related Chemicals by Microcoulometry, ASTM International, West Conshohocken, PA, 2018.